 |
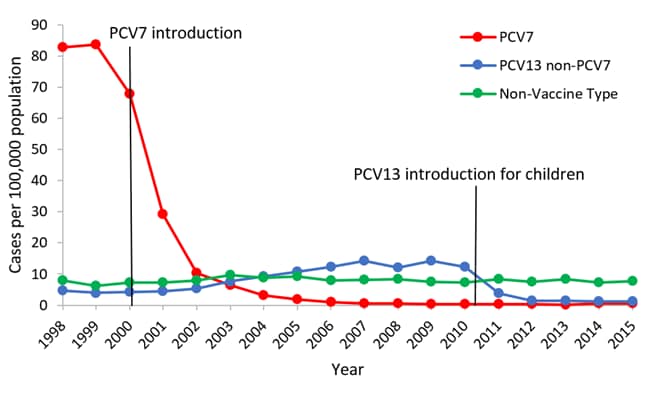
| Rates of invasive pneumococcal disease among children aged <5 years, 1998-2010* *Active Bacterial Core Surveillance data 1998-2010, unpublished |
In this issue of our on-going Vaccine Corner series, we take a look at
Pneumococcal disease.
One of the most common causes of pneumonia (an infection of the lungs) is the bacteria pneumococcus [pronounced "NEW mo ka kus"]. Besides causing pneumonia, this bacteria can cause other types of infections too, such as ear infections, sinus infections, meningitis and bacteremia. There are several strains of Pneumococcus bacterium and fortunately there is a vaccine (Prevnar-13) which protects against 13 of the most dangerous strains. Dr. McAllister agrees with the expert recommendations that all children receive the Prevnar vaccine at 2-months, 4-months, 6-months and 15-months.
Disease Description
The general name for infection with pneumococcus is pneumococcal disease. Before routine use of pneumococcal conjugate vaccine, the burden of pneumococcal disease among children younger than 5 years of age was significant. An estimated 17,000 cases of invasive disease (the most dangerous types of infections including: pneumonia, meningitis, joint or bone infections and bacteremia) occurred each year, of which 13,000 were bacteremia without a known site of infection and about 700 were meningitis. An estimated 200 children die every year as a result of invasive pneumococcal disease. Of children younger than 5 years old who get pneumococcal meningitis, about 1 out of 20 dies of the infection and others may have long-term problems such as hearing loss or developmental delay and 1 out of 100 children with bacteremia die from the infection. Although not considered an invasive disease, an estimated 5 million cases of acute otitis media occur each year among children younger than 5 years of age.
Effectiveness of the Vaccine
Data from the CDC suggests that the use of pneumococcal conjugate vaccine has had a major impact on the incidence of invasive disease among young children. The overall incidence of invasive disease among children younger than 5 years of age decreased from approximately 99 cases per 100,000 during 1998-1999 (before the vaccine) to 21 cases per 100,000 in 2008. The reductions in incidence resulted from a 99% decrease in disease caused by the seven serotypes in the first pneumococcal conjugate vaccine (PCV7) and serotype 6A, a serotype against which PCV7 provides some cross-protection. The decreases have been offset partially by increases in invasive disease caused by serotypes not included in PCV7.
A new vaccine (PCV13) was introduced in 2010 to offer protection against more strains of pneumococcus, leading to an even lower incidence of disease.
Risk of Vaccine
All immunizations have a risk of side effects, but most of them are mild and serious side effects are very rare.
Local reactions (such as pain, swelling or redness) following PCV13 occur in up to half of recipients. Approximately 8% of local reactions are considered to be severe (for example tenderness that interferes with limb movement). Local reactions are generally more common with the fourth dose than with the first three doses. Fever (higher than 100.4°) within 7 days of any dose of vaccine was reported for 24%-35% of children. High fever (104 or above) was reported in less than 1% of vaccine recipients. Nonspecific symptoms such as decreased appetite or irritability were reported in up to 80% of recipients.
Certain rare adverse events that were observed with use of the older PCV7 vaccine, and are considered possible with the newer PCV13 vaccine, although none of them were observed in the PCV13 clinical trials. These events include a coma like episode, apnea, anaphylactic reaction, erythema multiforme (a serious whole body rash), injection-site dermatitis, and swollen lymph nodes near the injection site. All of these events were rare and a causal relation of these events to vaccination is unknown.