|
Vitamin D Metabolism Dysregulation Causes Low Serum 25(OH)D
In the healthy individual, the complex interplay between innate and adaptive immunity cooperates to mount an appropriate response to infection through regulation of the vitamin D endocrine system.[1] Theoretically, the immune system detects and responds to the presence of cell wall deficient (CWD) bacteria by producing more 1,25(OH)2D to activate the VDR and express the crucial endogenous antimicrobial peptides (AMPs) which enable the innate immune system to target intracellular pathogens.[2] Renal production of 1,25(OH)2D is tightly self-regulated, with the end product down-regulating its own further production.
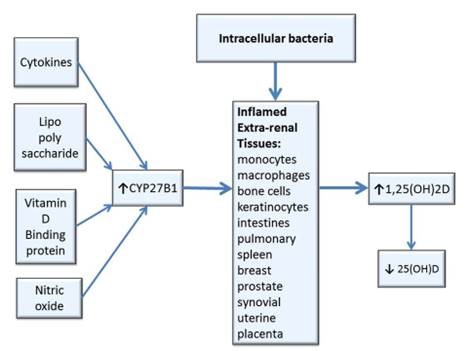
In contrast, extra-renal tissues (e.g., uterine decidua and placenta, bone cells, keratinocytes, colon, breast, prostate, spleen, melanoma cells, synovial, and pulmonary monocytes and macrophages, etc.) which produce 1,25(OH)2D are regulated by cytokines (e.g., interferon-gamma), lipopolysaccharide (an inflammatory molecule), nitric oxide (NO is given off by dying bacteria) and intracellular VDBP (the precursor for the principal macrophage activating factor (MAF)), which activate the enzyme CYP27B1 to stimulate conversion of 25(OH)D to 1,25(OH)2D.[3] Data suggest that local synthesis of 1,25(OH)2D may be a preferred mode of response to antigenic challenge in many tissues and locally synthesized 1,25(OH)2D has the potential to spill-over into the general circulation.[4] This extra-renal production of 1,25(OH)2D in tissues infected with intracellular bacteria can result in an excess production of 1,25(OH)2D which may contribute to depletion and low levels of 25(OH)D.[5]
Because extra-renal production of 1,25(OH)2D is primarily dependent on the availability of 25(OH)D, supplementation with vitamin D to increase 25(OH)D may promote the production of 1,25(OH)2D in non-renal tissues that are sites of intracellular infection and result in hypervitaminosis-D.[6]Sunlight appears to play a part in this process. Vanderschueren et al. observed seasonal variations in 1,25(OH)2D at all levels of 25(OH)D and concluded that sunlight exposure appears to have an influence on 1,25(OH)2D very similar to that of 25(OH)D.[7] The skin (dermal fibroblasts and keratinocytes possess VDR) has the capacity to synthesize 1,25(OH)2D, and represents an important target tissue for 1,25(OH)2D.[8] If keratinocytes in the skin are infected, natural regulation of photosynthesis may be thwarted and solar energy may overstimulate cellular activity, resulting in an increase in cutaneous production of vitamin D3, 25(OH)D and 1,25(OH)2D following sun exposure.
When nucleated cells are parasitized by CWD bacteria, extra-renal production of 1,25(OH)2D increases, the kidneys lose control of 1,25(OH)2D production, and pro-hormone 25(OH)D decreases due to rapid conversion to 1,25(OH)2D. The following mechanisms are thought to be responsible:
* Inflammatory cytokines activate CYP27B1 (formerly 1a-hydroxylase), an enzyme that causes more 25(OH)D to be converted to 1,25(OH)2D.[9]
* The microbial-repressed VDR can't transcribe CYP24A1 (formerly 24-hydroxylase), an enzyme that breaks down excess 1,25(OH)2D.[10]
* Excess 1,25(OH)2D binds the pregnane X receptor( PXR), to inhibit conversion of vitamin D3 to 25(OH)D so 25(OH)D is down-regulated.[11]
* 1,25(OH)2D inhibits the hepatic synthesis of 25(OH)D.[12]
Thus, low 25(OH)D may be a consequence of the inflammatory process. More studies are concluding that suboptimal circulating levels of vitamin D appear to be caused by the disease process. Waldrun et al. found serum 25(OH)D was decreased following an acute inflammatory insult (i.e., orthopedic surgery) and concluded hypovitaminosis D may be the consequence rather than cause of chronic inflammatory diseases.[13] Ferder et al. stated: ...there may be a relationship between inflammatory processes induced by chronic overstimulation of the renin angiotensin system (RAS) and the worldwide vitamin D deficiency... In fact, the pandemic of vitamin D deficiency could be the other face of increased RAS activity, which could potentially cause a lower activity or lower levels of Vitamin D.[14]
References
1. Norman A. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Amer J Clin Nutr. Aug 2008;88(2):491S-499S.
2. Rasmussen SB, Reinert LS, Paludan SR. Innate recognition of intracellular pathogens: detection and activation of the first line of defense. APMIS. May 2009(117(5-6)):323-37.
3. Dusso AS, Kamimura S, Gallieni M, et al. gamma-Interferon-induced resistance to 1,25-(OH)2D3 in human monocytes and macrophages: a mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab. 1997;82(7):2222-32.
4. Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007 Mar;103(3-5):316-21.
5. Edfeldt K, Liu PT, Chun R, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A. Dec 2010;107(52):22593-8.
6. Lambert PW, Stern PH, Avioli RC, et al. Evidence for extrarenal production of 1 alpha ,25-dihydroxyvitamin D in man. J Clin Invest. Mar 1982;69(3):722-5.
7. Vanderschueren D, Pye SR, O'Neill TW, et al. Active vitamin D (1,25-dihydroxyvitamin D) and bone health in middle-aged and elderly men: the European Male Aging Study (EMAS). J Clin Endocrinol Metab. Mar 2013;98(3):995-1005.
8. Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. Jul 2007;16(7):618-25.
9. Bikle DD. Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep. Jul 2009;7(2):58-63.
10. Jones G, Prosser DE, Kaufman M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch Biochem Biophys. Jul 2012;523(1)(9-18):9-18.
11. Xu Y, Hashizume T, Shuhart MC, et al. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1alpha,25-dihydroxyvitamin D(3): implications for drug-induced osteomalacia. Mol Pharmacol. Jan 2006;69(1):56-65.
12. Bell NH, Shaw S, Turner RT. Evidence that 1,25-dihydroxyvitamin D3 inhibits the hepatic production of 25-hydroxyvitamin D in man. J Clin Invest. Oct 1984;74(4):1540-4.
13. Waldron JL, Ashby HL, Cornes MP, et al. Vitamin D: a negative acute phase reactant. J Clin Pathol. Mar 2013;[Epub ahead of print].
14. Ferder M, Inserra F, Manucha W, Ferder L. The world pandemic of Vitamin D deficit could possibly be explained by cellular inflammatory response activity induced by the renin angiotensin system. Am J Physiol Cell Physiol. Jan 2013;[Epub ahead of print].
|