|
Upcoming Events
| |
Barcelona, Spain
June 2, 2016
University of Linz Austria
August 24-27, 2016
Submit an abstract!
Durham, NC
Sept. 29-30, 2016
Submit an abstract.
Juan-les-Pins, France
Oct. 17-20,2016
Abstract deadline extended until 6/5/16.
|
|
|
|
We're Hiring!
- Biologist I
- Quality Assurance Associate
- Study Director
|
|
| Altria | | ARDF | | Avon | | BAT | | Caudalie | | Colgate-Palmolive | | IFER | | Johnson & Johnson | | Kimberly-Clark | |
L Brands
| | M.A.C. Cosmetics | |
Mary Kay
| | PETA | | The Procter & Gamble Company | | SC Johnson & Son | | Unilever |
|
|
 |
|
| EPA Continues Efforts to Incorporate Alternative Approaches to Traditional Testing |
In a recent Letter to Stakeholders, Jack Housenger, Director of the EPA's Office of Pesticide Programs (OPP), outlines ways in which EPA's OPP plans to reduce the number of animals used in its traditional "6-pack" studies. OPP's activities in three areas - eye irritation, skin sensitization, and dermal toxicity - are covered in detail, along with the potential for the use of the Globally Harmonized System (GHS) to facilitate the adoption of alternative methods. These efforts are welcome news to the regulated pesticide industry, as well as to the many additional proponents of in vitro and in silico safety assessment methods.
IIVS has played an integral role in laying the groundwork for such an ambitious program. The EPA's first major policy change to incorporate non-animal methods came about from a multi-year cooperative program between EPA, IIVS, industry stakeholders and The Accord Group. Their efforts resulted in a policy allowing an alternative testing strategy for the assessment of eye irritation hazard for anti-microbial cleaning products, as well as conventional pesticide products on a case-by-case basis. To provide industry with more details about EPA OPP's efforts to reduce the use of animals and to describe IIVS' current efforts to address the "6-pack" endpoint of skin irritation, IIVS has scheduled a webinar for June 14, 2016. Dr. Anna Lowit, Senior Scientist, OPP's Health Effects Division, will provide an overview of EPA's programs to modernize the "6-pack" and provide suggestions on how stakeholders and EPA can cooperate to accelerate progress toward the animal-reduction goals. Dr. Rodger Curren of IIVS will describe IIVS' previous activities in developing the current non-animal strategy for eye irritation, and recent investigations into in vitro methods to assess skin irritation.
|
|
IIVS Workshop Explores Exposure and Dosimetry Considerations for Non-Animal Testing
|

IIVS brought together stakeholders from industry, government and academia to discuss considerations for exposure and dosimetry for non-animal testing during its 2.5-day workshop, "In Vitro Exposure Systems and Dosimetry Assessment Tools for Inhaled Tobacco Products", held April 4-6, 2016 in Bethesda, MD. Uniquely focused on in vitro testing, the workshop is the only known event of its kind.
Attendees, including toxicologists from industry, the FDA Center for Tobacco Products, the National Center for Toxicological Research and the US Environmental Protection Agency, heard presentations, attended poster sessions and breakout groups to gain a deeper understanding of the issues involving exposure and dosimetry of aerosols to cells and tissues in culture. Topics included the current status of in vitro to in vivo correlations, whole tobacco smoke and E-cigarette aerosol/vapor constituents, in vitro exposure systems, dosimetry approaches, the exposure microenvironment, and promising technologies that may advance science in these areas.
|
|
View the Latest IIVS Research
|
View our posters presented at recent meetings, including SOT 2016, our latest respiratory toxicology workshop, and the Pan-American Conference on Alternatives.
SOT 2016 (March 2016)
Effect of Acclimation Conditions on 3D Human In Vitro ALI Airway Models: Exposure-Induced Inflammatory Response and Sampling Location - Specific Results
Combining In Silico and In Vitro Methods to Improve the Accuracy of Skin Sensitization Predictions for Chemicals
Correlation of Two In Vitro EpiOcular Test Methods and Consumer Eye Irritation Data for Cleaning Products
Evaluation of New Solvents for the Use in the Multi-Dose Reconstructed Human Epidermis (RhE) Phototoxicity Assay
In Vitro Exposure Systems and Dosimetry Assessment Tools for Inhaled Tobacco Products (IIVS Workshop, April 2016)
Digital Dispensing onto 3D Airway Tissue: Delivery of Patterned, Picoliter Volumes for Direct Apical Surface Coverage with Precise Quantities of Test Materials In Vitro Exposure Systems and Dosimetry Assessment Tools for Inhaled Tobacco Products
Pan-American Conference for
Alternative Methods (April 2016)
The Assessment of Alternate Solvents for Use in the 3T3 Neutral Red Uptake (NRU) Phototoxicity Assay and the Influence of Solvents on the Prediction of Phototoxic Potential of Amiodarone
Evaluation of the Validated In Vitro Skin Irritation Test (OECD TG 439) for the Assignment of EPA Hazard Categories
Mimicking Metabolism Using Human Microsomes in Two In Vitro Assays in Order to Predict the Sensitization Potential of Pro-haptens
Improving the Accessibility and Efficiency of the Human Cell Line Activation Test
|
New Collaboration with M.A.C. Cosmetics
|
We are thrilled to welcome M.A.C. Cosmetics as a new partner in our efforts to eliminate the use of animal testing worldwide by increasing the use and acceptance of non-animal test methods. Through the generous support of M.A.C. and other companies we are able to promote the use of alternative methods and provide educational and training opportunities, such as lectures, workshops, and hands-on training sessions to scientists from countries that still rely on animal testing.
To learn more about our International Outreach Program, please contact Erin Hill at ehill@iivs.org.
|
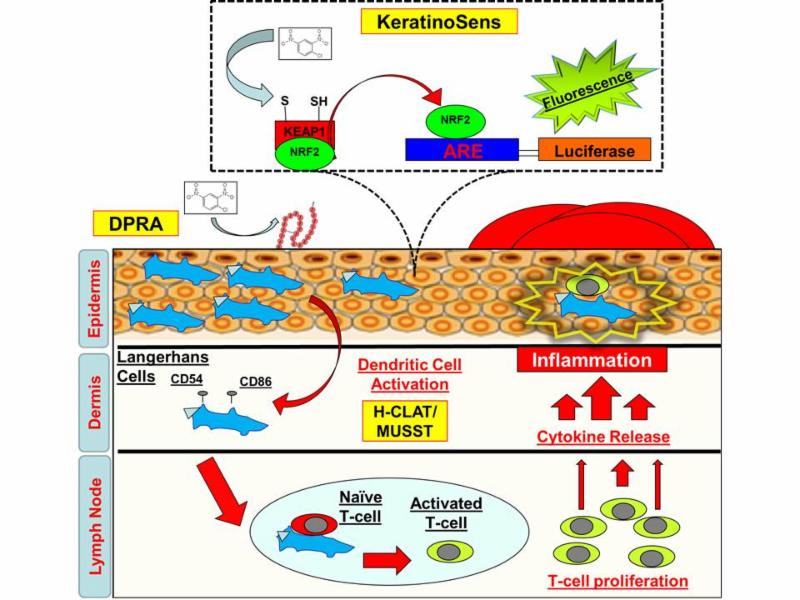
How do you assess
skin sensitization
effectively
using in vitro
methods ?
|
|
IIVS Recognizes Martin Stephens at Pan American Conference
|
|
 IIVS CEO, Rodger Curren, and President, Erin Hill, presented IIVS CEO, Rodger Curren, and President, Erin Hill, presented
Dr. Martin L. Stephens with an award recognizing his dedication to non-animal testing and his
long-time support of IIVS, including service on its Science Advisory Panel. The award was presented during the Pan-American Conference for Alternative Methods that was held April 12-14 in Baltimore.
Dr. Stephens is a Senior Research Associate with the Center for Alternatives to Animal Testing (CAAT) of the Johns Hopkins Bloomberg School of Public Health.
|
|
IIVS Study Director Emilia Costin to Present at the Institute of Biochemistry of the Romanian Academy
|
IIVS Study Director, Dr. Gertrude-Emilia Costin, will present a lecture entitled " In Vitro Testing Systems and Strategies Used in Toxicology. Current Status and Emerging Trends" as part of the Institute of Biochemistry of the Romanian Academy Seminar Series on July 1, 2016. More info can be found at www.biochim.ro/.
|
|
|