NMA Calls for Greater Protection
of Black Infants Against RSV
The National Medical Association (NMA) continues to advocate for increased awareness, education and protection of infants with respect to respiratory syncytial virus (RSV). RSV, a respiratory virus that infects the lungs and breathing passages, was discovered in 1956 and has since been recognized as one of the most common causes of childhood illness.
Since the release in 2010 of the landmark RSV Consensus Panel Paper by the NMA and the National Black Nurses Association, the NMA has been a relentless advocate for at-risk infants and has called for a conservative, research-based approach to prevention, diagnosis and treatment. In recent years there has been controversy surrounding dosing regimens and recommendations, but the NMA has been consistent in its call for protecting at-risk infants. The NMA has repeatedly reached out to the American Academy of Pediatrics, voicing grave concerns, providing expert opinion on any changes to the prophylaxis regimen, and offering unique guidance from the African American health prospective. The NMA has also continued to increase awareness and education about RSV in our communities and among physicians.
This special newsletter is part of the NMA's ongoing efforts to keep the nation's infants safe from the potential risks of RSV.
|
 Data Presented at the 2015 NMA's Annual Convention Data Presented at the 2015 NMA's Annual ConventionOn August 3, 2015, at the NMA's 113th Annual Convention and Scientific Assembly in Detroit, AstraZeneca presented the initial results of SENTINEL1, an ongoing observational study of respiratory syncytial virus-confirmed hospitalizations (RSVHs) among US infants born at 29-35 weeks gestational age (wGA) not receiving immunoprophylaxis (IP). The goal of the SENTINEL1 study is to assess the burden of severe RSV disease among preterm infants 29-35 wGA, following recent guidance that recommends against the use of IP for these infants. Initial results are based on data of all eligible infants with an RSVH, irrespective of enrollment status.
|
From the Centers for Disease Control
|
 Seasonal Trends Seasonal Trends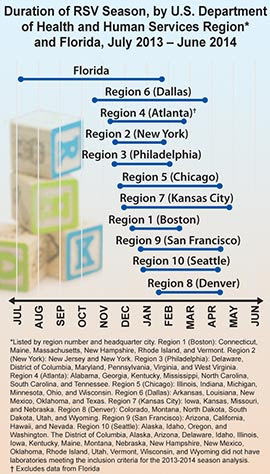 Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections among young children in the United States and worldwide. Most infants are infected before 1 year of age, and virtually everyone gets an RSV infection by 2 years of age. Respiratory syncytial virus (RSV) is the most common cause of lower respiratory tract infections among young children in the United States and worldwide. Most infants are infected before 1 year of age, and virtually everyone gets an RSV infection by 2 years of age.
Each year, on average, in the United States, RSV leads to- 57,527 hospitalizations among children younger than 5 years old;
- 2.1 million outpatient visits among children younger than 5 years old; and
- 177,000 hospitalizations and 14,000 deaths among adults older than 65 years.
In the United States and other areas with similar climates, RSV infections occur primarily during fall, winter, and spring.
The CDC analyzes data on RSV activity at the national, regional, and state levels, collected by a surveillance system called the National Respiratory and Enteric Virus Surveillance System (NREVSS).For 2013 to 2014, the RSV season onset ranged from late October to late January, and season offset ranged from late January to early April in all 10 U.S. Department of Health and Human Services (HHS) regions, except Florida. Florida has an earlier RSV season onset and longer duration than the rest of the country (see figure).
Seasonal patterns remained consistent with previous years.
Surveillance Systems Information on cases and outbreaks of RSV infection is collected in the United States using the National Respiratory and Enteric Virus Surveillance System (NREVSS). This is a voluntary, laboratory-based surveillance system that was established in 1989 to monitor trends in several viruses, including RSV. NREVSS tracks the number of RSV tests that are done by participating laboratories and the proportion that are positive, by specimen type, location, and when they were collected. Serotyping, demographic data, and clinical data are not reported. Data from NREVSS provides information to public health officials and healthcare providers about the presence of RSV in their communities.
|
 Clinical Description of RSV and Diagnosis Clinical Description of RSV and Diagnosis
|
In Infants
RSV infection can cause a variety of respiratory illnesses that sometimes cause fever. RSV infection most commonly causes a cold-like illness, but can also cause bronchitis, croup, and lower respiratory infections like bronchiolitis and pneumonia. Of every 100 infants and young children with RSV infection, 25% to 40% will show signs of pneumonia or bronchiolitis. Premature infants, very young infants, and those with chronic lung or heart disease or with weakened immune systems have a greater chance of having a more severe infection such as a lower respiratory tract infection. Infection without symptoms is rare among infants.
Infants with a lower respiratory tract infection typically have a runny nose and a decrease in appetite before any other symptoms appear. Cough usually develops 1 to 3 days later. Soon after the cough develops, sneezing, fever, and wheezing may occur. In very young infants, irritability, decreased activity, and apnea may be the only symptoms of infection.
Most otherwise healthy infants who are infected with RSV do not need hospitalization. Those who are hospitalized may require oxygen, intubation, and/or mechanical ventilation. Most improve with supportive care and are discharged in a few days.
In Adults
Symptomatic RSV infections may occur in adults, particularly in healthcare workers or caretakers of small children. Disease usually lasts less than 5 days, and symptoms are usually consistent with an upper respiratory tract infection and can include a runny nose (rhinorrhea), sore throat (pharyngitis), cough, headache, fatigue, and fever, but some high-risk adults, such as those with certain chronic illnesses or immunosuppression, may have more severe symptoms consistent with a lower respiratory tract infection, such as pneumonia.
|
Laboratory Testing
Several different types of laboratory tests are available for diagnosis of RSV infection. Rapid diagnostic assays performed on respiratory specimens are available commercially. Most clinical laboratories currently utilize antigen detection tests, and many supplement antigen testing with cell culture. Compared with culture, the sensitivity of antigen detection tests generally ranges from 80% to 90%. Antigen detection tests and culture are generally reliable in young children but less useful in older children and adults. Because of its thermolability, the sensitivity of isolation in cell culture
from respiratory secretions can vary among laboratories. Experienced laboratorians should be consulted for optimal results.
RT-PCR assays are now commercially available for RSV. The sensitivity of these assays often exceeds the sensitivity of virus isolation and antigen detections methods. Use of highly sensitive RT-PCR assays should be considered, particularly when testing older children and adults because they may have low viral loads in their respiratory specimens.
Serologic tests are less frequently used for routine diagnosis. Although useful for seroprevalence and epidemiologic studies, a diagnosis using paired acute- and convalescent-phase sera to demonstrate a significant rise in antibody titer to RSV cannot be made in time to guide patient care.
|
|
 NMA Consensus Panel Report NMA Consensus Panel Report
The NMA and the National Black Nurses Association (NBNA) convened a Consensus Panel to define the key needs in the area of respiratory syncytial virius (RSV) with respect to African Americans and other minorities. Findings and recommendations are included in the RSV Consensus Panel Report.
|

Physicians Urge RSV Protection for Black Infants
by Susanne Tropez-Sims, MD  "The Lives of Black Infants Matter!" the National Medical Association declared in a recent email update to members and colleagues. The statement referenced 2014 American Academy of Pediatrics guidelines, which limit preventative treatment against deadly respiratory syncytial virus (RSV) to only premature infants born before 29 weeks. Because black babies are more likely than white babies to face RSV risk factors, the AAP's restrictions disproportionately affect them.
I applaud the NMA's stance. It's time that both black health care providers and their non-black colleagues demand better protection for this vulnerable population.
Which Infants are More Vulnerable to RSV?
 RSV, with cold-like symptoms that can develop into serious breathing complications, bronchiolitis and wheezing, isn't uncommon. But premature infants' lungs and immune systems aren't always equipped to fight it off. So for these fragile babies, symptoms may warrant hospitalization and ventilator support. Not all of them survive. In fact, RSV causes 90,000 hospitalizations and 4,500 deaths per year in children 5 years of age and younger. It's 10 times more deadly than the flu. RSV, with cold-like symptoms that can develop into serious breathing complications, bronchiolitis and wheezing, isn't uncommon. But premature infants' lungs and immune systems aren't always equipped to fight it off. So for these fragile babies, symptoms may warrant hospitalization and ventilator support. Not all of them survive. In fact, RSV causes 90,000 hospitalizations and 4,500 deaths per year in children 5 years of age and younger. It's 10 times more deadly than the flu. No vaccine exists, but preventative treatment called "palivizumab" can help. For years, pediatric care providers have used palivizumab at their discretion to treat premature infants born before 37 weeks gestation.
Now, however, the AAP's revised guidelines tie providers' hands by limiting palivizumab access to only preterm infants born before 29 weeks. Insurance companies across the country have adopted these guidelines, pricing families out of RSV protection by eliminating reimbursement for treatment.
This policy shuts out the majority of preterm babies born in the United States every year. But it affects black babies in particular. Black babies are more likely than white babies to be born prematurely. And they are more likely to encounter the risk factors associated with RSV, such as low rates of breastfeeding, crowded living conditions, and contact with school-aged siblings and environmental pollutants.Without access to preventative RSV treatment, black babies face these risks unprotected. As a result, too many will also face RSV. To address the impending risks faced by black babies, the NMA held a landmark consensus panel on RSV. The panel included the nation's leading experts on pediatric care, particularly among African Americans. And it summarized its findings in a peer-reviewed consensus panel report, which advocated several responses to AAP's restrictive guidelines. Its priority recommendations includes calling for the AAP's Committee on Infectious Diseases (COID) to provide data supporting the dosing it now recommends. In addition, the report calls for the Centers for Disease Control to make RSV a reportable disease. Better reporting could offer more accurate data on the incidence of RSV and the demographics of the patients it affects. The NMA has also endorsed alternative guidelines issued by the National Perinatal Association. This organization's members stand by the Food and Drug Administration's approved use of palivizumab for infants 36 weeks gestation and younger. Broader access would allow health care providers once again to recommend palivizumab on a patient-by-patient basis. I urge the AAP COID to take NPA's guidelines and NMA's recommendations to heart. RSV season began in November for many regions of the country, andseveral states have already reported higher than usual rates of RSV. The lives of black babies - the lives of all babies - must be protected.
Susanne Tropez-Sims, MD is a practicing pediatrician in Nashville, Tenn. She is former chair of the National Medication Association's Pediatric Section and a member of the Alliance for Patient Access.
|

|
|
STAY CONNECTED  
|
|
|
|
|