|

FACT #1
Did you know that the FDA has recently recalled some commonly used household products? On January 5, 2011, the same company, the Triad Group, based in Hartland, Wisconsin, issued a voluntary nationwide recall of all lots of alcohol prep pads, alcohol swabs, and alcohol swabsticks because of potential microbial contamination.
According to the release, the recall was initiated because of concerns of contamination with Bacillus cereus. An MSNBC.com report indicates that a customer reportedly used an alcohol wipe on their 2-year-old son, who later died. The affected alcohol prep pads, alcohol swabs, and alcohol swabsticks were distributed in the United States, Canada, and Europe. They can be identified by having Triad Group listed as the manufacturer or by one of the following manufacturer names: Cardinal Health, PSS Select, VersaPro, Boca/Ultilet. Moore Medical, Walgreens, CVS, and Conzellin.
FACT #2
Researchers have found that past and current sun exposure and serum  vitamin D levels are independently associated with a reduced risk for a central nervous system first demyelinating event (FDE). Demyelination is a process that damages the myelin sheath of neurons. This process impairs the conduction of signals in the affected nerves and can be the first steps in the development of the disease multiple sclerosis. This study supports past research that shows vitamin D deficiency, potentially associated with lack of sun exposure, can contribute to MS. vitamin D levels are independently associated with a reduced risk for a central nervous system first demyelinating event (FDE). Demyelination is a process that damages the myelin sheath of neurons. This process impairs the conduction of signals in the affected nerves and can be the first steps in the development of the disease multiple sclerosis. This study supports past research that shows vitamin D deficiency, potentially associated with lack of sun exposure, can contribute to MS.
FACT #3
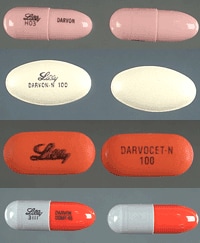
An estimated 10 million patients have used the pain reliever propoxyphene and were sent scrambling to doctors' offices when it was recently pulled from the market. "Propoxyphene is the worst drug in history," Ulf Jonasson, doctor of public health, from the Nordic School in Gothenburg, Sweden. "No single drug has ever caused so many deaths," Dr. Jonasson said.
 | | Clinicians are now prescribing analgesic alternatives to propoxyphene. |
Propoxyphene was banned in the United Kingdom 5 years ago because of its risk for suicide. It was taken off the market in Europe in 2009 over concerns about fatal overdoses and now in the United States for arrhythmias.
Also known as pethidine, Demerol was the first synthetic opioid synthesized in 1932 as a possible antispasmodic agent. Its analgesic
properties were recognized later. For much of the 20th century, pethidine has been the opioid of choice for many physicians treating acute and chronic severe pain.
"The writing has been on the wall for both of these drugs," Dr. Fraifeld said. "With adverse events, prescription abuse
increasing, and questionable effectiveness, this isn't innocuous."
Propoxyphene was first developed by Eli Lilly and later sold to Xanodyne Pharmaceuticals, which marketed the drug under the brand names Darvon and Darvocet.
New study results showed propoxyphene puts patients at risk for potentially serious or even fatal heart rhythm abnormalities. |